With high-tech ceramics, rubber, plastics, catalysts, environmentally friendly materials, aerospace evolving material, a magnesium oxide crystal material, in particular material applications high purity oxygen (MgO content of not less than 98%) of the more widely. For example, it is used to treat patients with hyperacidity and duodenal ulcer, and is used as a high-temperature annealing separator in the manufacture of silicon steel for the manufacture of electron tubes, filters, color filters, filters, and the like. In addition, as a sensitive high-efficiency catalyst and a good doping material, high-purity magnesium oxide is widely used in industrial catalysis and material modification and preparation of high-performance composite materials. There have been many methods for preparing high-purity magnesium oxide, such as magnesite (dolomite) carbonization, brine (seawater)-lime (ammonia), brine (seawater)-carbon, and magnesium salt direct pyrolysis. .
The molten salt method uses one or several low melting salts as a reaction medium to complete the synthesis reaction in a high temperature molten salt, and then dissolves the salt with a suitable solvent, and is filtered and washed to obtain a synthesized product which is oxidized at a high melting point. It is widely used in the fields of powders and electronic ceramic powders and other functional powder materials. The molten salt method has the advantages of simple process, low synthesis temperature, short holding time, low cost, stable and uniform chemical composition of the synthesized powder.
The different molten salt systems of MgO powder prepared by molten salt method were compared. It was found that NaCl-KCl salt has moderate melting point and relatively stable performance. NaCl and KCl are dissolved in water during washing, and the filtrate is dried to obtain NaC1, KC1 and other salts. The class is recyclable and is an excellent reaction medium. When the NaN0 3 -KN0 3 salt is used as the reaction medium, like the direct pyrolysis of the magnesium salt, a corrosive gas is generated during the reaction, which is not suitable for industrial production. However, the lower melting point of NaN0 3 -KN0 3 salt is beneficial to the analysis of the reaction process of the raw material system in the molten salt, and then the reaction mechanism is discussed. Therefore, Mg0 2 , CaCO 3 , NaN03 and KN0 3 are used as raw materials to prepare Mg0 powder. body.
First, the experiment
(1) Raw materials
The anhydrous magnesium chloride, calcium carbonate, sodium nitrate, potassium nitrate and absolute ethanol used in the experiment were all analytically pure.
(2) Preparation of magnesium oxide powder
MgCl 2 , CaCO 3 and NaN0 3 , KN0 3 are placed in a milled mill according to the ratio of 1.1..1..2..2, and the raw materials are uniformly mixed and ground to -0.074 mm, and heat-treated at 550 ° C for 3 h. It is soaked in water, washed, filtered under reduced pressure, dried at 110 ° C, and then heat treated at 600 ° C for 3 h.
(III) Analysis of reaction mechanism
As CaCO 3 and MgCl 2 -CaCO 3 -NaN0 3 -KN0 3 of TG-DSC curve, the thermal analysis of the feed during the reaction; The TG-DSC curve, the raw material calcined at different temperature and time, determine product composition, melt analysis The reaction mechanism of preparing magnesium oxide by salt method.
(4) Characterization
The samples were subjected to thermal effect analysis using a German NETZSCH STA449/6/G type thermogravimetry-differential scanning integrated thermal analyzer.
The product was subjected to phase identification using an X'Pert Pro type X-ray diffractometer manufactured by Philips, The Netherlands.
The morphology and size of the powder were observed by a Nova400 Nano SEM type field emission scanning electron microscope manufactured by Philips, The Netherlands.
Second, the results and discussion
(1) Analysis of composition and morphology of samples

Figure 1 is an XRD pattern of the S 11 sample and the S 12 sample, wherein the S 11 sample is a heat treatment of the raw material at 550 ° C for 3 h, washed with water and dried at 110 ° C, and the S 12 sample is S 11 The product was heat treated at 600 ° C for 3 h.
It can be seen from Fig. 1 that the raw material is heat-treated at 550 ° C for 3 h, and the precursor after washing with water is mainly magnesium hydroxide, wherein a small amount of magnesium oxide is not hydrolyzed, and the magnesium hydroxide is decomposed into magnesium oxide by heat treatment at 600 ° C for 3 h.

Figure 2 Sample TEM
(a) S 11 ; (b) S 12
2 is an SEM image of the S 11 sample and the S 12 sample. It can be seen from Fig. 2 that the magnesium hydroxide precursor is mainly in the form of layered morphology, irregular in shape, uneven in size distribution, thickness between 0.03 and 0.05 μm, diameter between 0.2 and 1.0 μm; The magnesium oxide has a granular morphology, a uniform size distribution, and a particle size of between 0.2 and 0.5 μm.
Table 1 shows the results of chemical composition analysis of the S 12 sample. From Table 1, high purity magnesium oxide powder prepared to meet pharmaceutical, metallurgy, industrial catalysis, quantum devices, microelectronics industry requirements.
Table 1 Results of chemical composition analysis of S 12 samples (mass fraction) /%
Mg0 | CaC0 3 | A1 2 0 3 | Si0 2 | Fe 2 0 3 | IL |
98.82 | 0.52 | 0.10 | 0.09 | 0.06 | 0.41 |
(2) Analysis of reaction mechanism
Figure 3 is a TG-DSC curve of CaCO 3 and MgC1 2 -CaCO 3 -NaN0 3 -KN0 3 starting materials.
It can be seen from Fig. 3(a) that the weight loss from 700 ° C to 800 ° C is 37.08%, CaCO 3 is decomposed into CaO and CO 2 , and the corresponding DSC curve has an endothermic peak at 769.2 ° C.

It can be seen from Fig. 3(b) that the weight loss from room temperature to 400 °C is 18.90%. In this temperature range, the raw material loses all physical water and structural water, and NaN0 3 -KNO 3 melts, and there are three endothermic peaks on the corresponding DSC curve; The weight loss from 400 ° C to 530 ° C is 8.10%. There is an endothermic peak at 490.5 ° C on the corresponding DSC curve. Decomposition reaction may occur in this temperature range; the weight loss from 530 ° C to 700 ° C is 23.20%, and the corresponding DSC curve is at 660.4. °C has an endothermic peak, and decomposition reaction may occur in this temperature range; after the temperature is greater than 700 °C, the weight loss continues to increase, mainly because the molten salt accelerates evaporation at high temperature. Referring to Figure 3 (a), there is no endothermic peak of CaCO 3 decomposition, indicating that CaCO 3 has completely reacted before 700 °C.
Figure 4 is an XRD pattern of the sample. The M 11 sample is a product which is heat treated at 320 ° C for 48 h, washed with water and dried at 110 ° C; M l2 sample is heat treated at 320 ° C for 360 h, washed with water and dried at 110 ° C; M 14 test The sample was heat treated at 900 ° C for 3 h, and the XRD pattern of the product was washed with absolute ethanol. It can be seen from Fig. 4 that the raw materials are heat-treated at 320 ° C for 48 h, and the products dried by water washing at 110 ° C are mainly magnesium carbonate and dolomite and a small amount of magnesium hydroxide; the raw materials are heat-treated at 320 ° C for 360 h, and after washing at 110 ° C. The dried product is mainly magnesium carbonate; the raw materials are heat-treated at 900 ° C for 3 h, and the products are all treated with magnesium oxide after washing with absolute ethanol.

Combining the XRD patterns of S 11 and S 12 samples, the reaction mechanism of MgO powder prepared by molten salt method using MgCl 2 , CaCO 3 , NaNO 3 and KNO 3 as raw materials is as follows:
1. The substitution reaction of Mg 2+ and Ca 2+ in the molten salt environment is related to the reaction temperature and reaction time.
MgCl 2 â†â†’Mg 2+ +2Cl -
xMg 2+ +CaCO 3 →MgxCa 1-x CO 3
When x < 0.5. The product is a displacement type solid solution of calcium carbonate. When x=0.5, the product is CaMg(C0 3 ) 2 . When 0.5<x<1, the product is a mixture of CaMg(C0 3 ) 2 and MgCO 3 , with the reaction continuously. for, when x = 1, the product is MgC0 3.
2. Decomposition of magnesium carbonate.
MgC0 3 →Mg0+C0 2 ↑
3. Hydrolysis of magnesium oxide during water washing.
Mg0+H 2 0→Mg(OH) 2
4. Decomposition of magnesium hydroxide.
![]()
Third, the conclusion
(1) In the process of preparing magnesium oxide from MgCl 2 -CaC0 3 -NaN0 3 -KN0 3 raw materials, Mg 2+ and Ca 2+ are displaced in molten salt environment to form intermediate products such as dolomite and magnesium carbonate. Continuously, the dolomite is finally converted into magnesium carbonate; the heat-treated magnesium carbonate at 550 °C is decomposed into magnesium oxide, and after being immersed in water, the magnesium oxide is hydrolyzed to form magnesium hydroxide, and the heat-treated magnesium hydroxide at 600 ° C is decomposed into magnesium oxide.
(2) The magnesium hydroxide precursor is an irregular layered morphology with uneven size distribution, the thickness is between 0.03 and 0.05 μm, and the diameter is between 0.2 and 1.0 μm; the magnesium oxide of the product is granular and sized. The distribution is relatively uniform, and the particle size is between 0.2 and 0.5 μm.
Batch Waste Tyre Pyrolysis Plant
Pyrolysis Plant is used for recycling waste tire, waste plastic, waste rubber to fuel oil. According to the actual situation in every country and district, we developed different models of Batch Pyrolysis Plants for waste tires, rubber and plastic with daily capacity 5 -10 tons.

Flowchat of Batch Waste Tyre Pyrolysis Plant
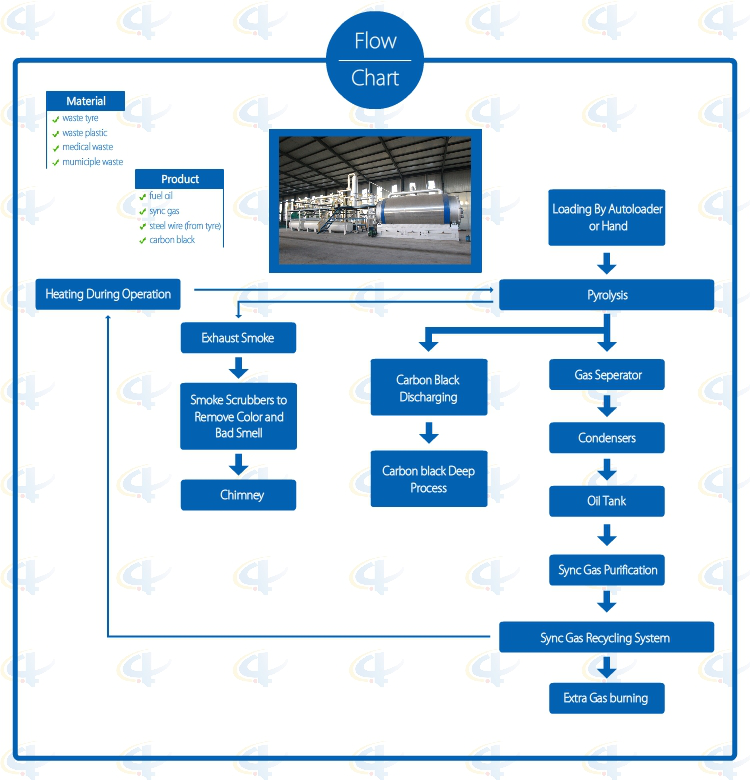
Advantages of Batch Waste Tyre Pyrolysis Plant
1. Full open door design: Speedy loading in and steel pulling out; Easily cooling down after one batch finished, saving time; No leaking with special high temperature flexible graphite packing.
2. Unique Craft Condensers: High condensing efficiency with more oil output. Good quality oil, longer lifetime, and easy to clean.
3. National Patent Unique Smoke Scrubbers: More efficient removal of the acid gas and dust of the smoke by neutralization, purification and absorption, environmental friendly without pollution.
4. National Patent Carbon Black Discharging System: Speedy fully enclosed Auto-discharging under high temperature, avoiding carbon black pollution, saving time.
5. Automatic Submerged welding technology, ultrasonic nondestructive testing, both manual and the automatic safety devices.
6. Sync Gas Recycling System: Fully burned after recycling and utilization, saving fuel and preventing pollution.
7. Direct Heating System: Enlarging heating square to lengthen the lifespan of the reactor and easy to control the temperature.
8. National Patent, unique heat insulation shell; high efficiency temperature keeping, excellent energy-saving effect.
Technical Parameter of Batch Waste Tyre Pyrolysis Plant
|
NO. |
ITEM |
PROJECT |
|
|
1 |
Equipment Model |
XY-7 |
XY-8 |
|
2 |
Door Model |
Full Open Door |
Full Open Door |
|
3 |
Suitable Raw Materials |
Rubber/Plastic Products |
Rubber/Plastic Products |
|
4 |
Structure |
Horizontal Type Revolves |
Horizontal Type Revolves |
|
5 |
Reactor Size |
Φ2200*6000mm Φ2600*6600mm |
Φ2200*6000mm Φ2600*6600mm |
|
6 |
Capacity for One Batch |
5-6Mt; 8-10Mt |
5-6Mt; 8-10Mt |
|
7 |
Oil Yield of Tires |
40%-45% |
40%-45% |
|
8 |
Work Pressure |
Normal Pressure |
Normal Pressure |
|
9 |
Reactor Rotation Speed |
0.4R/M |
0.4R/M |
|
10 |
Fuels Choice |
Coal, Wood |
Coal, Wood, Gas, Oil |
|
11 |
Power |
18KW/H |
18-25KW/H |
|
12 |
Cooling Method |
Water Cycling |
Water Cycling |
|
13 |
Type of Drive |
External Annular Gear |
External Annular Gear |
|
14 |
Heating Method |
Direct |
Direct |
|
15 |
Type of Installation |
With Foundation |
With Foundation/Integrated Base |
|
16 |
Noise dB(A) |
≦85 |
≦85 |
|
17 |
Operation Mode |
Intermittent Operation |
Intermittent Operation |
|
18 |
Total Weight(MT) |
25-40 |
25-40 |
|
19 |
Installation Space Required |
30m*10m |
30m*10m |
|
20 |
Manpower |
3~4/batch |
3~4/batch |
|
21 |
Shipment |
Ф2200×6000=1*40HC+1*40FR Ф2600×6600=2*40HC+1*40FR |
Ф2200×6000=1*40HC+1*40FR Ф2600×6600=2*40HC+1*40FR |
Batch Waste Tyre Pyrolysis Plant
Batch Waste Tyre Pyrolysis Plant,Waste Tyre Pyrolysis Plant,Rubber Pyrolysis Recycling Plant
Shangqiu Jinpeng Industrial Co., Ltd. , https://www.recyclings.nl